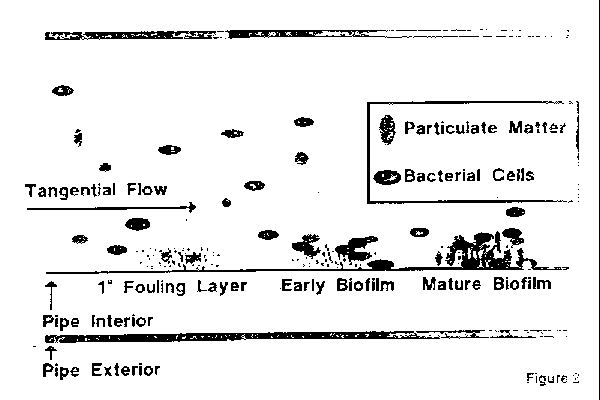
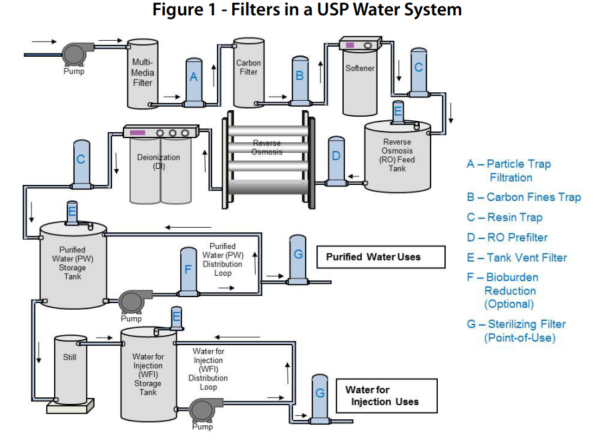
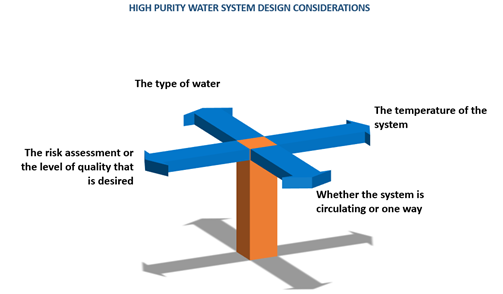
Each plastic fusion weld received a verification label in accordance with fda documentation requirements for weld tracking and upon completion the system was flushed and pressure tested with di water in compliance with customer specifications.
High purity water systems fda.
4 titled design concepts for the validation of a water for injection system.
Contact fda follow fda on facebook follow fda on twitter view fda videos on youtube subscribe to fda rss feeds fda homepage contact number 1 888 info fda 1 888 463 6332.
Content current as of.
Monitoring and validation of high purity water systems with the lal test for pyrogens t j.
Previous chapter table of contents.
Food and drug administration 10903 new hampshire avenue silver spring md 20993 1 888 info fda 1 888 463 6332 contact fda.
4 titled design concepts for the validation of a water for.
These guidelines of ora within fda are not addressed to the industry but to the fda inspectors.
4 titled design concepts for the validation of a water for injection system the introduction provides guidance and states that validation often involves the use of an appropriate challenge.
Novistsky pharmaceutical engineering march april 1984.
The result was a contiguous leak free system in the ceilings and walls.
A basic reference used for the validation of high purity water systems is the parenteral drug association technical report no.
Here you will find guidelines for the inspection of water systems both for purified water and for water for injection wfi.
The guides to inspection are however a useful aid.
A basic reference used for the validation of high purity water systems is the parenteral drug association technical report no.
A basic reference used for the validation of high purity water systems is the parenteral drug association technical report no.